

Rise Up
Introducing RISE UP,
a NEW clinical study to
assess pain crises & anemia
in sickle cell disease
RISE UP (2021‑001674‑34) is a
phase 2/3, double-blind,
randomized, placebo-controlled,
multicenter clinical trial.1
This clinical study will evaluate
the efficacy and safety of
mitapivat in the treatment of
sickle cell disease for participants
16 years of age and older.1
Rise up clinical
study design1
PHASE 3 (Now Enrolling)
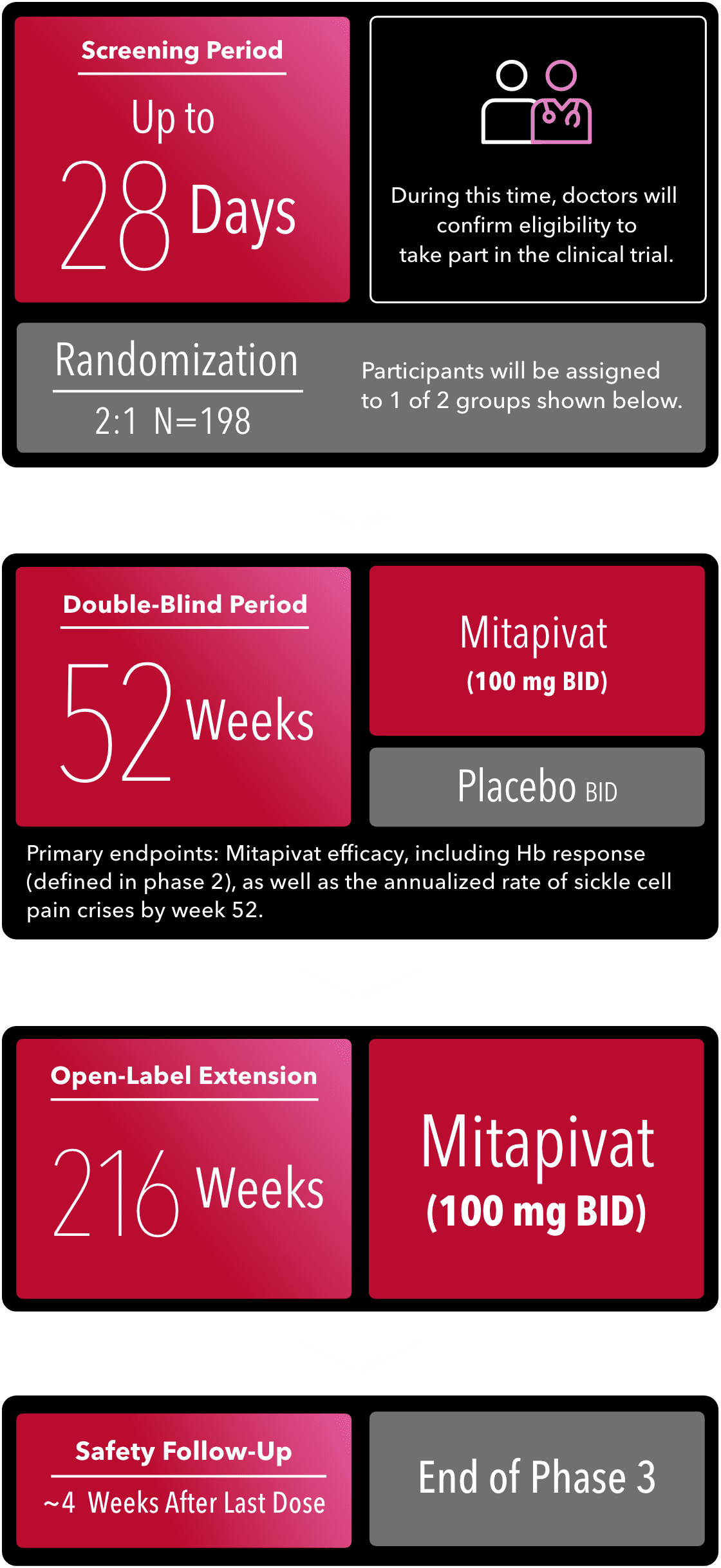
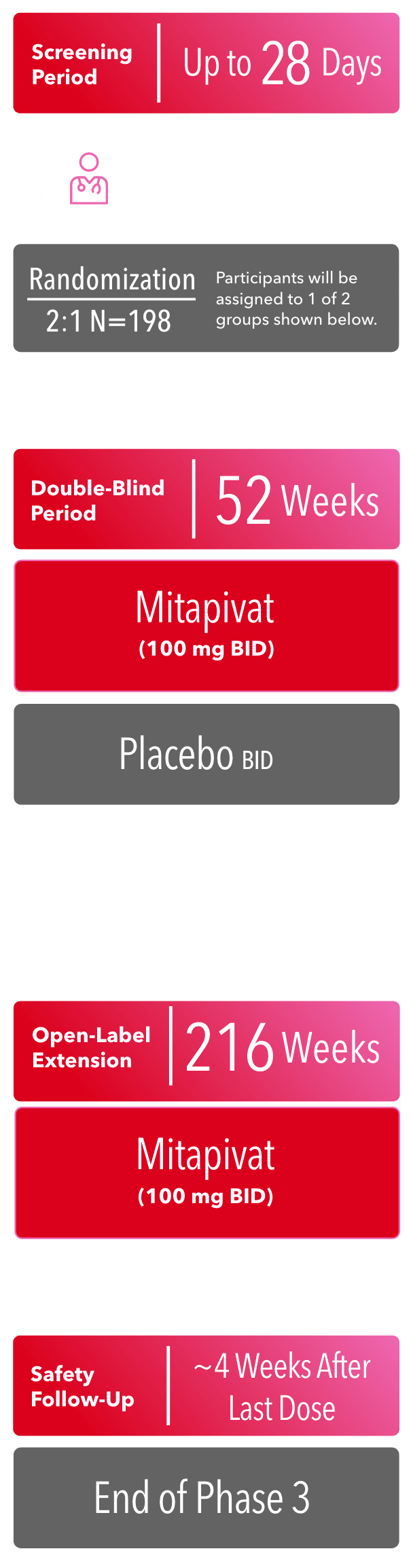
BID=twice daily; Hb=hemoglobin.
KEY INCLUSION CRITERIA1
- Age 16 years or older, with a documented diagnosis of sickle cell disease (HbSS, HbSC, HbS/β0 thalassemia, HbS/β+ thalassemia, or other sickle cell syndrome variants)
- At least 2 and no more than 10 sickle cell pain crises in the past 12 months prior to informed consent
- Defined as acute episodes of pain, acute chest syndrome, priapism, or hepatic or splenic sequestration
- If taking hydroxyurea, the hydroxyurea dose must be stable for at least 90 days before randomization
KEY EXCLUSION CRITERIA1
- Pregnant or breastfeeding
- Receiving regularly scheduled transfusions
- Hepatobiliary disorders, significant liver disease, gallbladder disease, or severe kidney disease
- Prior exposure to gene therapy or prior bone marrow or stem cell transplantation
- Currently receiving voxelotor, crizanlizumab, or L-glutamine
- Currently receiving treatment with hematopoietic-stimulating agents
- Taking strong CYP3A4/5 inhibitors or strong inducers of CYP3A4
PK activation supports red blood cell health
Mitapivat — the study drug in RISE UP — is an investigational, oral, allosteric activator of the PK enzyme1,3
PK enzyme activation may improve the health, energy, and lifespan of red blood cells (RBC) for patients with hemolytic anemias1
- Increasing ATP production, helping to match RBC energy need
- Decreasing 2,3-DPG which reversibly increases oxygen affinity for hemoglobin, potentially reducing sickling
- Maintaining antioxidants, thereby reducing cellular damage
References:
1. Data on file. Agios Pharmaceuticals, Inc.
2. Research!America. National public opinion poll. July 2017. Accessed October 18, 2023. https://www.researchamerica.org/wp-content/uploads/2022/07/July2017ClinTrialMinorityOversamplesPressReleaseSlidesFINAL_0-1.pdf
3. Howard J, Kuo KHM, Oluyadi A, et al. A phase 2/3, randomized, double-blind, placebo-controlled study of mitapivat in patients with sickle cell disease. Blood. 2021;138(suppl 1):3109.

